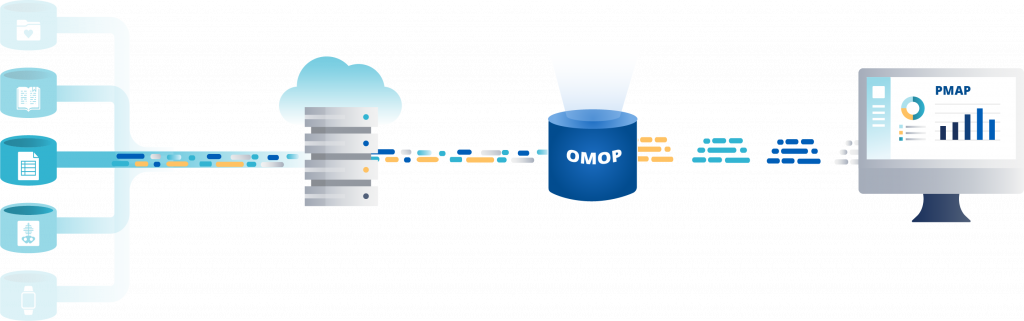
What is OMOP?
The Observational Medical Outcomes Partnership (OMOP) dataset on PMAP is a first-rank research resource representing the largest, most up to date, curated representation of patients receiving care at Johns Hopkins Medicine.
The OMOP Common Data Model (CDM) enables the capture of patients information in the same way across departments, institutions, and research partners around the globe.
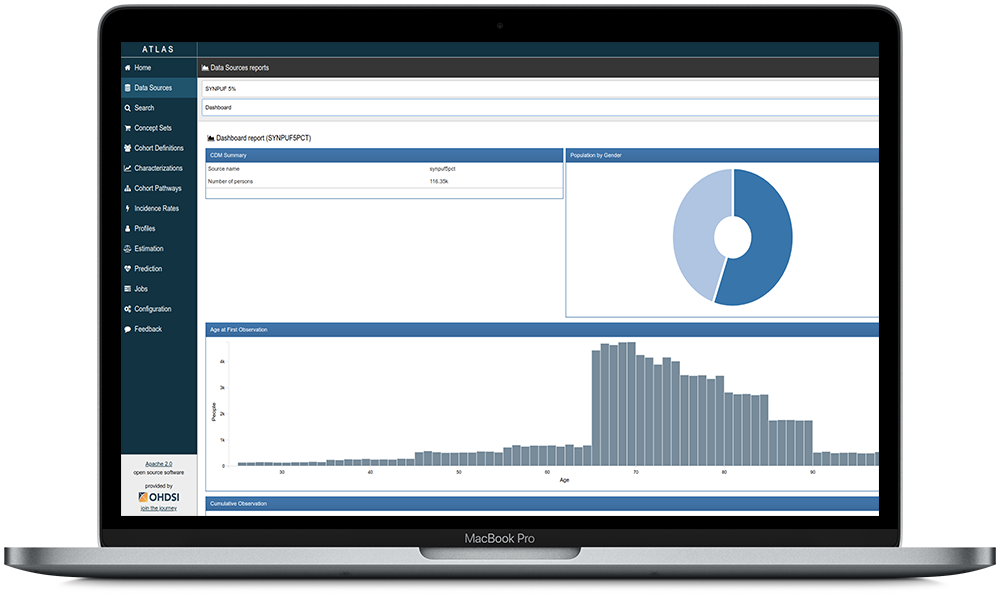
Why is OMOP important?
Transforming and Scaling Data
The Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) allows for reproducibility and collaboration across institutions. It is recognized as the industry gold standard both in the U.S. and around the globe. Researchers can access not only Johns Hopkins data using PMAP, our discovery and delivery platform, but can quickly scale to larger research studies since it uses standardized language and codes.
OMOP on PMAP can help you accelerate your clinical research.
500+ Million Patients
OMOP is a common data model widely adopted by medical institutions across the U.S. and around the world.

3,000 Data Quality Tests
OMOP is the most curated data standard using the Data Quality Dashboard.

Powerful Analysis Tools
Access to powerful tools for evidence generation: API Rstudio/Python, SQL via SAFE, Crunchr or Databricks

Observational Data
At Scale
OMOP enables the data transformation to be easily distributed and massively scaled.
 Johns Hopkins OHDSI Research Network
Johns Hopkins OHDSI Research Network
The Observational Data Sciences and Informatics (or OHDSI, pronounced “Odyssey”) research network is an international community using empirical methods to conduct observational and reproducible research. We are building an active, local OHDSI community of Johns Hopkins clinical researchers who are supporting each other to help take advantage of the global OHDSI community.
Links & Resources about data creation and quality
We’ve created tools to help you understand Johns Hopkins Medicine data
Note: Most of these tools are only accessible via SAFE Desktop
What our users say about OMOP

We’re using OMOP on PMAP to apply for extramural funding – including career development awards. In OMOP with ATLAS we can define cohorts and these cohorts are malleable. This has NIH reviewers and collaborators really excited – that you can adjust cohorts to answer a wide array of research questions while preserving data security and data quality.
Assistant Professor of Pediatrics

This is a movement towards accessible science. OMOP on PMAP is foundational to my PhD work. The Johns Hopkins OHDSI community, tools and resources allow me to address unanswered clinical questions by leveraging granular EMR data with the generalizability of the entire OHDSI network.
MD-PhD Candidate

Engaging OMOP on PMAP is a powerful example of: Think Global; Act Local. OMOP enables research at Johns Hopkins, while also allowing for observational research across institutions. OMOP provides the leverage to translate real-world data into precision medicine.
Instructor of Oncology
Frequently Asked Questions
- Here is a resource that provides an overview of the OMOP Common Data Model and all the tables in the CDM: https://ohdsi.github.io/TheBookOfOhdsi/CommonDataModel.html#CommonDataModel
- Free training in the CDM can be found on the EHDEN Academy (https://academy.ehden.eu/).
- To find out what data is available, refer to the Data Catalog of OMOP data set (https://datacatalog.pm.jh.edu/home).